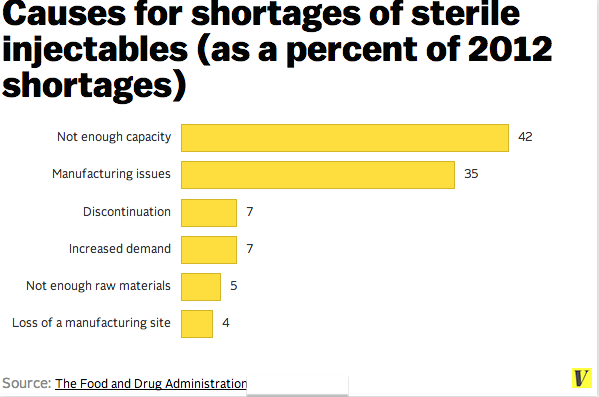
Over the past several years, drug shortages have continued to complicate the routine delivery of health care in the United States. The Food and Drug Administration (FDA) is currently tracking nearly 100 shortages resulting from a host of causes, such as the consolidation of markets and manufacturers, limited availability of raw materials, and quality problems resulting in product recalls and delivery interruptions (see Examples of Drug Shortages Listed on the FDA Website for examples).
1
Clinicians have become accustomed to these deficiencies, and they troubleshoot accordingly. Yet there are important implications for the quality and safety of patient care, since clinicians must regularly negotiate unfamiliar drug alternatives, concentrations, or dosing strategies. Shortages also have significant budgetary implications. For example, according to some of our colleagues, in 2012 doxycycline sold for a penny a tablet, yet because of a cascade of manufacturer shutdowns and discontinuations, the current price is at least $1.59 per tablet.
Current critical shortages of vecuronium, intravenous nitroglycerin, and lorazepam have required the hospitals where two of us work to adopt contingency measures, including, in one case, providing explicit, written direction about substitutions for intravenous nitroglycerin. Complicating the ability to substitute, however, are further shortages of potential replacement medications. Nicardipine and a component of nitroprusside drips, thiosulfate, are both in short supply.
The national shortage of normal saline, however, represents a new chapter in supply shortages.
2The administration of normal saline (and other crystalloids) is a basic underpinning of patient care. In many ways, it is more the lifeblood of hospital care than blood itself. As supply-chain disruptions have caused a shortage of saline, demand for alternatives has spiked, causing a ripple effect. As hospitals increase their product orders to compensate for the shortage, the backlog of orders awaiting delivery grows. The fact that health care facilities would have to ration the use of such a fundamental resource is perhaps the most significant demonstration yet of the importance of addressing these vulnerabilities.
So what should we do? Drug shortages provide the opportunity, if not the imperative, to apply emergency-management methods to the daily delivery of care. Using these “disaster-response” tools on a day-to-day basis offers a systematic, coordinated approach and also prepares institutions to deploy them more effectively during a large-scale disaster.
Four foundational elements of emergency management — mitigation, preparedness, response, and recovery — provide a framework that health care institutions can use to grapple with the everyday challenge of drug and other product shortages. Mitigation involves preventing the incident from occurring or blunting its impact through design or system changes. The FDA has taken proactive steps, in partnership with manufacturers and others, to prevent or reduce the effects of shortages. Through a range of approaches, such as early notification, expedited inspections, and regulatory flexibility, the FDA helped prevent 282 shortages in 2012 and 170 shortages in 2013. These successful efforts provide strong groundwork on which to build greater transparency and supply-chain monitoring into our daily systems, although a recent report of the Government Accountability Office suggests that there is still room for strengthening responses to drug shortages.
3
Preparedness activities involve making plans and obtaining adequate supplies. Health care institutions can take these steps by determining who has what authority (e.g., for purchasing or new vendor agreements), roles, and responsibilities for incident management during supply shortages; stockpiling vital supplies during times of no shortage; identifying backup vendors; and finding partners, such as other health care institutions, that can collaborate and share information and resources during a shortage.
During an active drug shortage or other crisis, the plans and partnerships developed during preparedness activities can be put to use. Primarily, the response can be managed through an incident-management system, a framework widely used by hospitals and emergency services that establishes leadership, clear roles, responsibilities, procedures, and communication channels for addressing the incident. Incident management is scalable and responsive to the size of the event. In the case of a shortage of normal saline, a hospital system could designate a pharmacy or administrative leader who would coordinate and be responsible for activities to address the shortage.
Many hospitals and health care coalitions are already taking this approach to respond to drug shortages. Pharmacy supervisors are often designated as incident managers. They maintain situational awareness by comparing any shortfalls in orders against current stock levels and expected monthly consumption levels, optimally providing early warning of a shortage situation. They can communicate these shortages to providers in terms of “red, yellow, green” triage categories denoting the degree of severity, taking into account factors such as the urgency of the shortfall and its probable effect on clinical practice. They can also build safeguards into the ordering of unfamiliar agents (through alerts incorporated into the electronic health record system) and their preparation (for instance, preparing them in the pharmacy rather than on the patient's hospital floor).
The collaboration involved in the incident-management process and the clarity that ideally results from it mean that clinicians are not forced to make ad hoc decisions that may be inconsistent with ethical frameworks or preferred strategies. Instead, uniform stewardship actions taken throughout the whole institution — or the whole community — such as sharing, conserving, substituting, adapting, reusing, and reallocating, can be used to address shortages.
These key stewardship actions are components of the Institute of Medicine's standards-of-care guidelines for catastrophic emergencies. Developed initially for planning for an H1N1 influenza pandemic, these guidelines provide foundational ethical and procedural guidance for the allocation of scarce resources during disasters. This guidance was recently adapted by the Association of State and Territorial Health Officials for use in drug-shortage events.
4,5
Incident management also promotes coordination of decision making with other health care facilities, systems, and partners in the community (including emergency medical services and public health agencies) by designating liaisons as the points of contact and coordination. During the recent saline shortages, for example, the Northern Virginia Hospital Alliance released a coalition-purchased disaster cache of intravenous fluids to member hospitals for their immediate use, stemming the need for restrictive conservation measures. Many hospitals offered guidance to providers on using alternative crystalloids or smaller bags, emphasizing oral hydration, enteral feeding, and reduced preoperative fasting times whenever possible. These policies are often shared with other facilities and through health care networks and response coalitions, resulting in consistent, community-wide practices.
Recovery should begin even during an organization's response to a shortage, through replenishment of consumed “emergency” supplies, with the goal of returning the facility to usual practices, if not better ones. For example, a retrospective review might lead to decisions to increase the backup for critical supplies when they are not in short supply or to revise plans for similar events in the future.
The shortage of normal saline highlights the way in which breaking even a single link in our fickle supply chain can have profound effects on routine health care delivery. Application of stewardship strategies for resource allocation tailored to the degree of deficit, coupled with the use of an incident-management system, will ensure a fair, informed, and structured response across this delivery continuum.
Finally, an added benefit to “practicing” these strategies during day-to-day incidents is that personnel will then be more comfortable with them when a full-scale disaster occurs. In other words, preparedness rests on the back of strong day-to-day systems. Daily use translates to disaster utility.
EXAMPLES OF DRUG SHORTAGES LISTED ON THE FDA WEBSITE.*
Atropine sulfate injection
Bupivacaine hydrochloride (Marcaine, Sensorcaine) injection
Calcium chloride injection
Calcium gluconate injection
Dobutamine hydrochloride injection
Epinephrine injection
Epinephrine (1 mg/ml, preservative-free)
Fentanyl
Heparin sodium injection
Lidocaine hydrochloride (Xylocaine) injection
Magnesium sulfate injection
Nitroglycerin in 5% dextrose injection
Sodium chloride (0.9%) injection bags